Study Finds that Microtubules are Effective Light Harvesters: Implications for Information Processing in Sub-Cellular Systems
A remarkable study on electronic energy migration in microtubules has revealed unexpected light-harvesting capabilities in these cellular structures [1]. Published in the journal ACS Central Science, the study "Electronic Energy Migration in Microtubules" by a coalition of researchers from multiple institutions—including Princeton, Stanford, Oxford, Arizona State University, the Indian Institute of Technology in Delhi, and others— have demonstrated that microtubules, cylindrical polymers of tubulin protein, can conduct electronic energy over distances of up to 6.6 nm, comparable to some photosynthetic complexes. The crystalline order of microtubules aligns light-harvesting amino acid chromophore subunits in close enough proximity to effect relatively long-range exciton energy transfer along the cytoskeletal filaments. The findings of the study demonstrated that after photoexcitation amino acid chromophores had resonant transfer of excitation energy along the microtubule comparable in efficiency to artificial light-harvesting systems, suggesting they are natural effective light harvesting macromolecular structures and can direct coherent exciton diffusion over distances much greater than what was previously presumed from first order calculations. This finding challenges previous assumptions about the quantum properties of biological systems and may have significant implications for our understanding of cellular processes, anesthetic mechanisms implicating microtubules in cognitive processes, macromolecular optoelectrical mechanics in cellular information processing, and the development of bio-inspired technologies.
What are Microtubules
Microtubules are dynamic, cylindrical filamentary structures composed of tubulin protein subunits, playing a pivotal role in maintaining cell shape, enabling intracellular transport, cellular motility, and facilitating chromosome segregation during cell division. These polymers are integral to the cytoskeleton, providing structural support and serving as tracks for motor proteins that transport cellular cargo and are therefore instrumental to internal cellular organization. Beyond their mechanical and motility functions, microtubules have been implicated in cellular signaling via multiple mechanisms [2, 3, 4, Microtubule-Actin Network Within Neuron Regulates the Precise Timing of Electrical Signals via Electromagnetic Vortices]. Their potential roles in orchestrating cellular information processing, which increasingly appears to be multitudinous—even potentially underlying cognitive processes—have become an area of focus for many researchers.
Recent studies have uncovered intriguing quantum properties within microtubules, particularly involving aromatic amino acid residues like tryptophan [5]. These residues are capable of participating in electronic energy transfer, a process that is essential for various cellular functions. Tryptophan, known for its unique fluorescence characteristics, acts as a key player in these quantum phenomena. It contributes to the formation of energy-conducting networks within the microtubule structure, facilitating long-range energy migration and potentially supporting quantum coherence. This discovery opens new avenues for exploring the intersection of quantum biology and cellular dynamics, offering insights into the fundamental processes that underpin life at the molecular level.
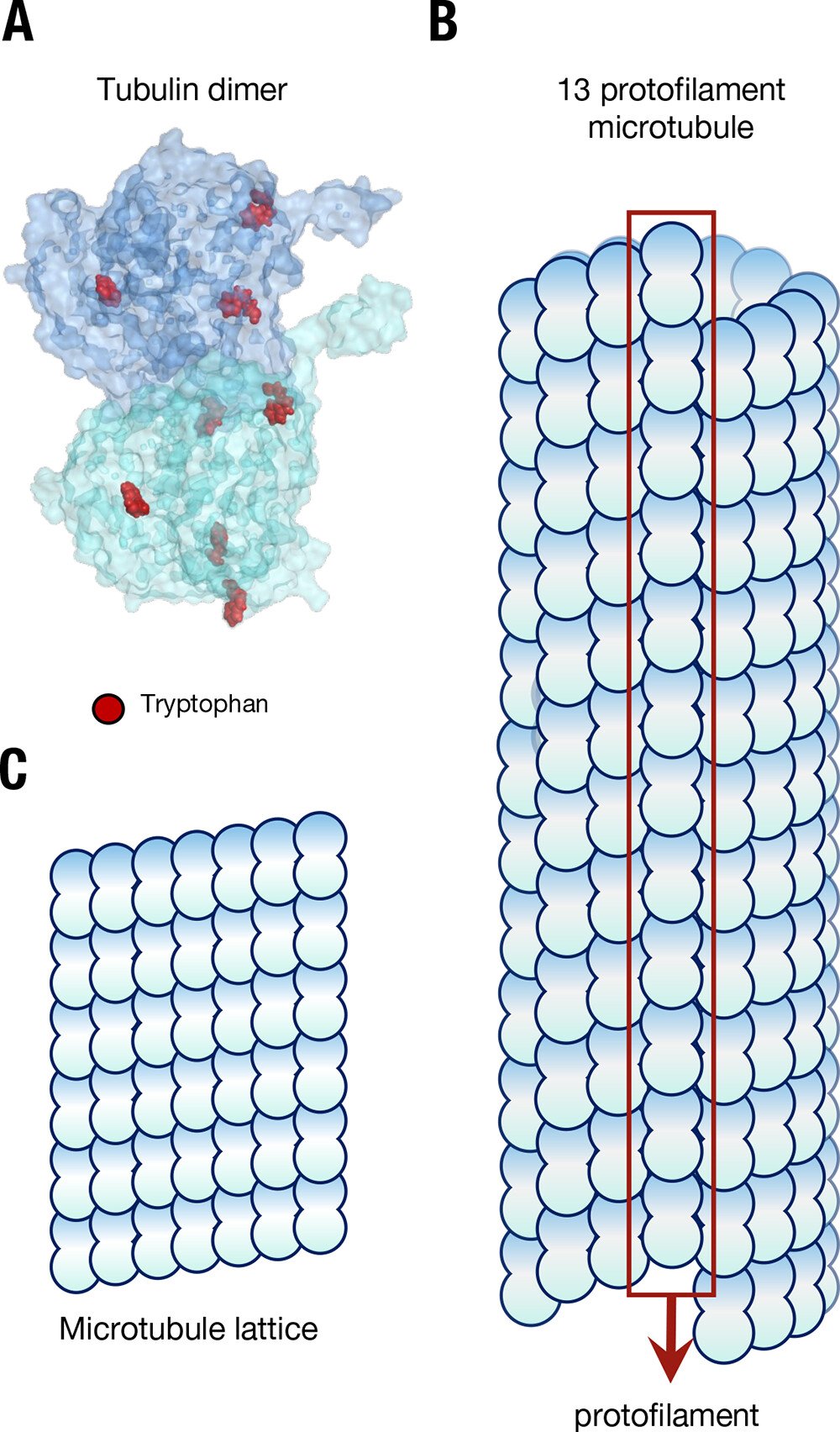
Aromatic amino acids like tryptophan are residues (i.e., subunits) of the protein tubulin (Figure 1, A) and when tubulin dimers are polymerized into helical protofilament microtubule crystalline arrays (Figure 1, B) the aromatic amino acid antennae resonators form coordinated networks, with some "mega-networks" involving up to 10,000 residues (see our article Long-range Collective Quantum Coherence in Tryptophan Mega-Networks of Biological Architectures).
Figure 1.
“The structure of microtubules forms a lattice of tubulin. (A) The tubulin dimer with tryptophan residues marked in red; the C- termini “tails” can be seen protruding from each monomer. (B) The structure of a microtubule, showing constituent arrangement of tubulin dimers, and the presence of a “seam”. (C) The repeating “lattice” of tubulin dimers in a microtubule.” Image and image description from [1] A. P. Kalra et al., “Electronic Energy Migration in Microtubules,” ACS Cent. Sci., vol. 9, no. 3, pp. 352–361, Mar. 2023, doi: 10.1021/acscentsci.2c01114.
The role of organic benzene/phenyl ‘pi electron resonance’ molecules, like tryptophan and tyrosine, as central in potential collective quantum coherent properties of microtubules has long been predicted by Stuart Hameroff, an anesthesiologist at the University of Arizona whom in collaboration with physicist and Nobel laureate Sir Roger Penrose formulated one of the first theories of consciousness involving subcellular dynamics and even quantum gravitational mechanisms [6, 7].
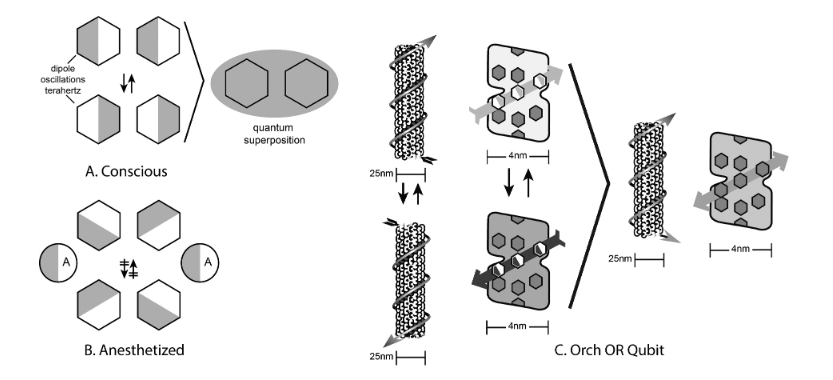
Hameroff and colleagues postulated that long-range coupling of the oscillating dipoles of aromatic amino acids residues in tubulin monomers of microtubules could process information in unique ways—such as massive parallel processing due to collective synchronization of dipole oscillators—and a proposed mechanism of orchestrated objective reduction (Orch-OR, Figure 2).
Figure 2.
“(a). Organic benzene/phenyl ‘pi electron resonance’ molecules couple, form oscillating dipoles, and quantum superposition. (b). Anesthetic gas molecules disperse dipoles, disrupt coherent oscillations, preventing consciousness. (c). The Orch OR qubit - Left: Collective dipoles oscillate in single tubulin, and along a helical microtubule pathway. Right: Quantum superposition of bothorientations in a tubulin pathway qubit.” Image and image description from [[8] S. Hameroff, “‘Orch OR’ is the most complete, and most easily falsifiable theory of consciousness,” Cognitive Neuroscience, vol. 12, no. 2, pp. 74–76, Apr. 2021, doi: 10.1080/17588928.2020.1839037.
Proposed nearly three decades ago, this theory has come to be known as the Orch-OR model, and while it has seen some criticisms during its long history, it has withstood the tests of both scrutiny and time and has recently seen wide-ranging empirical support via direct experimental observations and measurements of quantum properties of microtubules from multiple independent laboratories. One such experimental observation shedding light on the non-trivial quantum properties of microtubules is a recent study by an international research team that has found long-range electronic energy diffusion in the subcellular filaments, even confirming the disruption of such coupled electronic energy resonance transfer by anesthetic molecules, which was another prediction of Hameroff’s Orch-Or theory [9].
What was Found
The research team, led by Aarat P. Kalra and colleagues used tryptophan autofluorescence to probe energy transfer between aromatic amino acid residues in tubulin and microtubules. By studying how quencher concentration alters tryptophan autofluorescence lifetimes, they were able to quantify the extent of electronic energy diffusion in these structures.
Their results showed that aromatic amino acid residues called chromophores (chromophores are light sensitive antennas in biological macromolecules), like tryptophan and tyrosine, have robust coupling strengths over relatively long distances, with electronic energy transfer among coordinated residues occurring over approximately 6.6 nanometers for microtubules. This length of electronic energy diffusion is surprisingly high because conventionally microtubules were thought to play only structural and locomotive roles in the cell, and it is only recently that their photo-electronic processing behaviors, such as efficient electronic energy transfer, have been unequivocally identified. For comparison, chlorophyll a, a chromophore in the photosynthetic antenna complex, is specifically optimized for resonance energy transfer and absorbs photons with about 20 times the efficiency of tryptophan yet has a diffusion length of only 20-80 nm.
These findings were unexpected and challenge conventional models, such as Förster theory, which predicted electronic energy diffusion distances on the order of one nanometer for chromophore resonant transfer typical of inter-tryptophan dipole-dipole coupling, a significantly smaller distance than what was measured by Scholes et al.
Moreover, since the diffusion length of approximately 6.6 nm is on par with the size of a single tubulin dimer, which is roughly equivalent to the volume of a sphere of diameter 7.4 nm, it indicates that energy transfer between tryptophan residues could occur across a single tubulin dimer within a polymerized microtubule. As such, if a photoexcitation event occurred in a chromophore residue near an adjacent tubulin dimer, then it could be transferred along the microtubule crystalline lattice (Figure 3). Effectively resulting in coherent photon/exciton transfer, or electronic energy transfer along microtubule filaments, acting as veritable info-energy transmission filaments in subcellular macromolecular reticular networks.
Figure 3.
Schematic showing long-range energy transport along a microtubule. Image reproduced from [1].
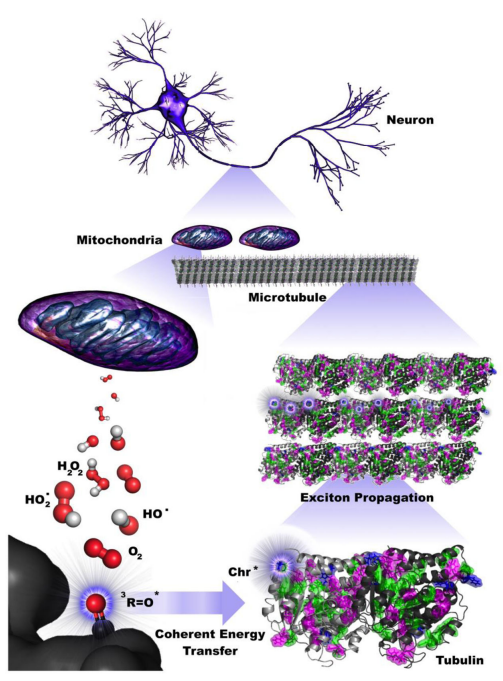
Photoexcitation events may occur continuously from reactive oxygen species production from mitochondria as described by Kurian et al., [10] (Figure 4), in which mitochondria are known to form helical filamentary networks with microtubules (Figure 5) [11].
Figure 4.
Coherent energy transfer in microtubule chromophore networks is stimulated by ultraweak photoemissions due to mitochondrial reactive oxygen species (ROS) production. Filamentous mitochondria are co-located with microtubules in the brain, suggesting that mitochondrial ROS production during respiratory activity may affect neuronal activity. Specific ROS (red and white), particularly triplet carbonyls (red and black), emit in the UV range, where aromatic networks composed of mainly tryptophan and tyrosine may be able to absorb and transfer this energy along the length of neuronal microtubules. The propagation of these excitons extends on the order of dendritic length scales and beyond, indicating that ultraweak photoemissions may be a diagnostic hallmark for neurodegenerative disease and have implications for aging processes. Image and Image description from [10] P. Kurian, T. O. Obisesan, and T. J. A. Craddock, “Oxidative Species-Induced Excitonic Transport in Tubulin Aromatic Networks: Potential Implications for Neurodegenerative Disease,” J Photochem Photobiol B, vol. 175, pp. 109–124, Oct. 2017, doi: 10.1016/j.jphotobiol.2017.08.033.
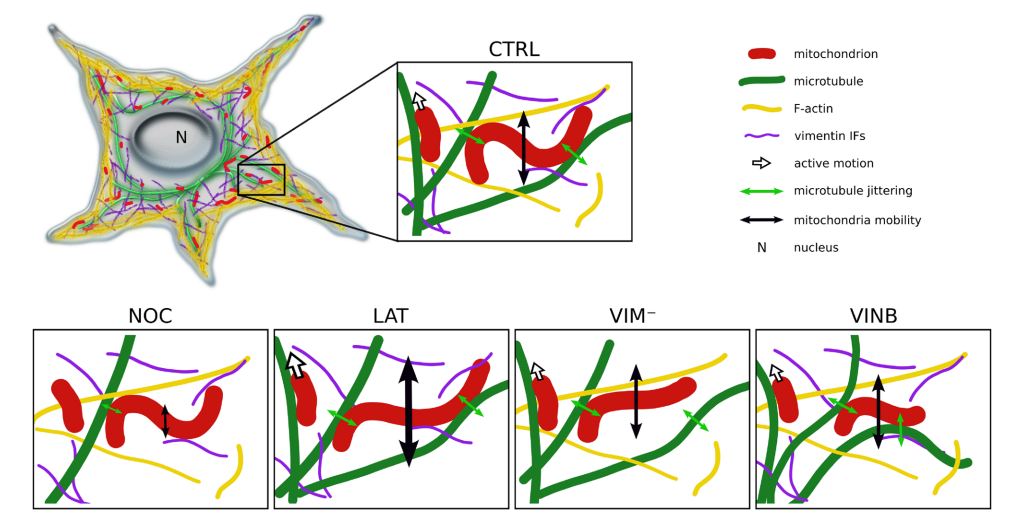
Figure 5.
Summary of the modulation of mitochondrial shape fluctuations and mobility by the cytoskeleton. Mitochondria are in close association with microtubules, being transported through them and modifying their shape as a consequence of the jittering transmitted by these filaments (green double arrows) and the interactions with F-actin and vimentin IFs, both of which would contribute to maintain mitochondria confined to microtubule network. Upon partial depolymerization of microtubules (NOC), both the mobility of the organelles (schematized with the black double arrows) and the mechanical force imposed on them decrease. Given the disruption of F-actin (LAT) and vimentin IFs (VIM−) networks, a predominance of elongated mitochondria is observed, suggesting that these filaments also modulate the organelles’ shape. F-actin depolymerization also results in increased mitochondrial mobility, suggesting that these filaments impose greater spatial confinement that restricts their motion. Perturbation of microtubule dynamics (VINB) decreases mitochondrial curvature and length compared to the control condition (All images created by A.B. Fernández Casafuz). Image and Image description from [11] A. B. Fernández Casafuz, M. C. De Rossi, and L. Bruno, “Mitochondrial cellular organization and shape fluctuations are differentially modulated by cytoskeletal networks,” Sci Rep, vol. 13, no. 1, p. 4065, Mar. 2023, doi: 10.1038/s41598-023-31121-w.
Interestingly, the researchers found that while diffusion lengths were influenced by tubulin polymerization state (free tubulin versus tubulin in the microtubule lattice), they were not significantly altered by the average number of protofilaments (13 versus 14). This suggests that the energy transfer properties are intrinsic to the tubulin structure rather than dependent on specific microtubule architectures.
How the Study was Performed
The researchers employed a multi-faceted approach to investigate electronic energy migration in microtubules. Their methodology included:
Steady-state spectroscopy: This technique was used to measure the absorption and fluorescence spectra of tubulin and microtubules under various conditions.
Time-correlated single photon counting (TCSPC): This advanced method allowed the team to measure tryptophan fluorescence lifetimes with high precision, providing crucial data on energy transfer dynamics.
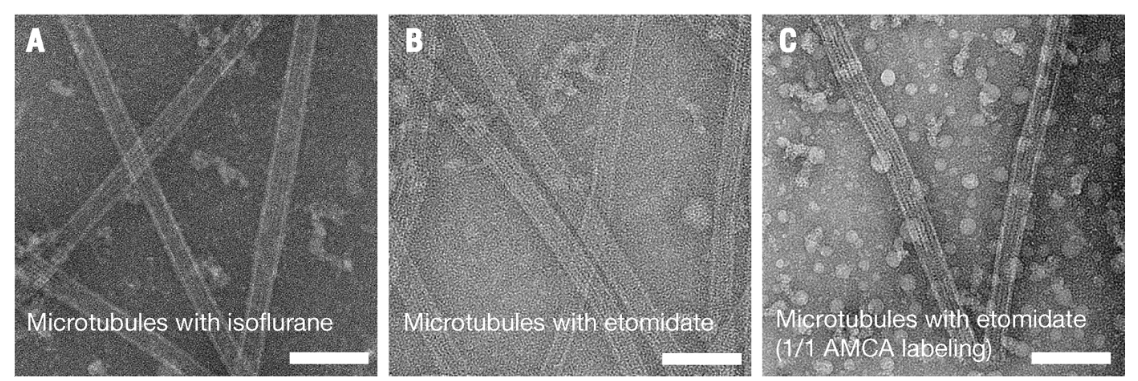
Negative stain electron microscopy: This imaging technique was used to confirm the polymerization states and structures of the tubulin assemblies studied (Figure 6).
Figure 6.
Tunneling electron microscopy validating microtubule polymerization in anesthetic containing solutions (A) isoflurane, (B) etomidate, (C) etomidate and microtubules polymerized using tubulin labeled with AMCA. Scale bars represent 100 nm. Image from [1].
In addition to these experimental methods, the team also performed computational simulations to model energy migration in microtubules. They created a computational microtubule model composed of 31 stacked tubulin rings and used it to calculate coupling strengths for energy transfer between tyrosine and tryptophan residues.
The researchers also investigated the effects of anesthetics on energy transfer in microtubules. They introduced etomidate and isoflurane into their assays and measured their impact on tryptophan fluorescence quenching.
Investigation of Anesthetic Action on Energy Transfer in Microtubules
The researchers conducted a detailed investigation into how anesthetics influence energy transfer within microtubules, since it has been a long-time theory of Hameroff and his collaborators that anesthetics work—at least in part— via inhibitory action of microtubule function, most probably via disrupting dipole-dipole coupling and hence halting resonance energy transfer and long-range collective coherence. By introducing anesthetics such as etomidate and isoflurane into their experimental assays, the research team were able to observe changes in tryptophan fluorescence quenching, a method used to assess the impact of these substances at a molecular level.
The researchers were able to empirically explore whether microtubules might facilitate quantum processes that are involved with cognitive processes and awareness. Anesthetics, known for their ability to induce unconsciousness, were found to interfere with energy transfer in microtubules, suggesting a possible link between microtubule dynamics and conscious states.
The findings propose that the disruption of energy transfer mechanisms by anesthetics could inhibit the quantum processes within microtubules necessary for consciousness.
Anesthetic drugs are effective in organisms ranging from paramecia, to plants, to primates (suggesting elements of proto-cognition are operable even in unicellular and aneural organisms) and are known to have targets in the cytoskeleton, ion channels, and mitochondria [12]. Moreover, several recent studies have implicated non-chemical quantum properties like nuclear spin and magnetic field effects on anesthetic potency [13], which again highlights the potential role of non-trivial quantum effects in microtubules that are verifiably affected by anesthetics.
The action of anesthetics on excitation energy transfer was found to alter tryptophan fluorescence lifetimes when tested via spectroscopic analysis. Introducing anesthetics, etomidate and isoflurane, into microtubule assays was shown to decrease diffusion lengths of excitation energy transmission, affect dipole-dipole interactions, reduce exciton diffusion, dampen electronic energy migration, interfere with long-range interactions, potentially inhibit dipole-based information processing, and therefore overall impacting the efficiency of electronic energy migration in microtubules. This behavior, now directly observed in experiment, supports the hypothesis [14] that long-range dipole-switching of aromatic residues for information processing is an active mechanism in the cellular cytoskeleton network.
Overall, this line of research opens new avenues for understanding how anesthetics modulate consciousness and highlights the significant role microtubules might play in cognitive functions.
Potential Insights to Glean from the Study
The findings of this study have far-reaching implications for our understanding of quantum effects in biological systems. For years, many experts believed that the biological environment was too "wet, noisy, and warm" for non-trivial quantum effects like long-range collective quantum coherence to occur. This study provides strong evidence to the contrary, demonstrating that life has indeed leveraged non-trivial quantum mechanical phenomena for its own benefit (note, there is the trivial sense in which quantum mechanics is operable in the biological system, e.g., determining electron orbital configurations and holding together molecules, non-trivial refers to QM effects apart from those that are obviously at play).
These results lend support to theories such as the Unified Spacememory Network proposed by Haramein and Brown [15], and the Orch-OR theory of Hameroff and Penrose. Both of these theories involve subcellular macromolecular assemblies like microtubules playing crucial roles in information processing and potentially in cognition and awareness.
The study also has implications for our understanding of anesthetic mechanisms. The finding that the presence of anesthetics like etomidate and isoflurane reduced exciton diffusion in microtubules is a direct indication that these optoelectrical cellular filaments and associated photon/exciton transfer dynamics are involved in information and energy processes correlated with consciousness. This observation aligns with theories that anesthetics may work by interfering with quantum coherence in neuronal microtubules.
As well, the exciton vibrational resonance energy transport is corroborative of similar studies like that by Geesink et al. in which quantum coherence and entanglement play an integral role in information processing dynamics by these subcellular structures. In a study Geesink and Schmieke found that microtubule frequencies comply with two proposed quantum wave equations of respective coherence (regulation) and decoherence (deregulation), that describe quantum entangled and disentangled states [16]. The research team also suggests that microtubules show a principle of a self-organizing-synergetic structure called a Fröhlich-Bose-Einstein state, in which the spatial coherence of the state can be described by a toroidal topology. They suggest that their study reveals an
informational quantum code with a direct relation of the eigenfrequencies of microtubules, stem cells, DNA, and proteins, that supplies information to realize biological order in life cells and substantiates a collective Fröhlich-Bose-Einstein type of behavior; further supporting the models of Tuszynski, Hameroff, Bandyopadhyay, Del Giudice and Vitiello, Katona, Pettini, Pokorny, and other prominent researchers who have posited non-trivial quantum properties of microtubules involved in cognition and awareness.
This study empirically demonstrates the long-range collective resonance of dipole oscillators in microtubules, implicating the cytoskeleton in information processing by utilizing quantum properties. By revealing the unexpected light-harvesting capabilities of microtubules, the research challenges traditional views of cellular structures and highlights their potential role in quantum coherence and information processing. This finding not only advances our understanding of the quantum realm within biology but also opens new avenues for exploring how the novel forms of matter found in the nanomachinery of life might be reverse engineered in bio-inspired technologies and perhaps even revealing something fundamental about the nature of sentience that is such a key characteristic of life and the living system.
References
[1] A. P. Kalra et al., “Electronic Energy Migration in Microtubules,” ACS Cent. Sci., vol. 9, no. 3, pp. 352–361, Mar. 2023, doi: 10.1021/acscentsci.2c01114.
[2] C. D. Velasco, R. Santarella-Mellwig, M. Schorb, L. Gao, O. Thorn-Seshold, and A. Llobet, “Microtubule depolymerization contributes to spontaneous neurotransmitter release in vitro,” Commun Biol, vol. 6, no. 1, pp. 1–15, May 2023, doi: 10.1038/s42003-023-04779-1.
[3] B. C. Gutierrez, H. F. Cantiello, and M. del R. Cantero, “The electrical properties of isolated microtubules,” Sci Rep, vol. 13, no. 1, p. 10165, Jun. 2023, doi: 10.1038/s41598-023-36801-1
[4] Singh, P., et al. "Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices." Symmetry, 2021, 13(5), 821.
[5] S. Eh. Shirmovsky and D. V. Shulga, “Quantum relaxation processes in microtubule tryptophan system,” Physica A: Statistical Mechanics and its Applications, vol. 617, p. 128687, May 2023, doi: 10.1016/j.physa.2023.128687.]
[6] Hameroff, S., & Penrose, R. "Orchestrated reduction of quantum coherence in brain microtubules: A model for consciousness." Mathematics and Computers in Simulation, 1996, 40(3), 453-480.
[7] S. Hameroff, “Consciousness, Cognition and the Neuronal Cytoskeleton – A New Paradigm Needed in Neuroscience,” Front. Mol. Neurosci., vol. 15, Jun. 2022, doi: 10.3389/fnmol.2022.869935.
[8] S. Hameroff, “‘Orch OR’ is the most complete, and most easily falsifiable theory of consciousness,” Cognitive Neuroscience, vol. 12, no. 2, pp. 74–76, Apr. 2021, doi: 10.1080/17588928.2020.1839037.
[9] Kalra, A. P., Hameroff, S., Tuszynski, J., Dogariu, A., Nicolas, Sachin, & Gross, P. J. 2022, August 14. Anesthetic gas effects on quantum vibrations in microtubules – Testing the Orch OR theory of consciousness. https://osf.io/zqnjd/ Date created: 2020-04-01 Last Updated: 2022-08-14.
[10] P. Kurian, T. O. Obisesan, and T. J. A. Craddock, “Oxidative Species-Induced Excitonic Transport in Tubulin Aromatic Networks: Potential Implications for Neurodegenerative Disease,” J Photochem Photobiol B, vol. 175, pp. 109–124, Oct. 2017, doi: 10.1016/j.jphotobiol.2017.08.033.
[11] A. B. Fernández Casafuz, M. C. De Rossi, and L. Bruno, “Mitochondrial cellular organization and shape fluctuations are differentially modulated by cytoskeletal networks,” Sci Rep, vol. 13, no. 1, p. 4065, Mar. 2023, doi: 10.1038/s41598-023-31121-w
[12] M. B. Kelz and G. A. Mashour, “The Biology of General Anesthesia from Paramecium to Primate,” Current Biology, vol. 29, no. 22, pp. R1199–R1210, Nov. 2019, doi: 10.1016/j.cub.2019.09.071.
[13] H. Zadeh-Haghighi and C. Simon, “Radical pairs may play a role in microtubule reorganization,” Sci Rep, vol. 12, no. 1, p. 6109, Apr. 2022, doi: 10.1038/s41598-022-10068-4.
[14] A. P. Kalra et al., “Anesthetic gas effects on quantum vibrations in microtubules – Testing the Orch OR theory of consciousness,” Apr. 2020, Accessed: Sep. 03, 2024. [Online]. Available: https://osf.io/zqnjd/
[15] Haramein, N., Brown, W. D., & Val Baker, A. "The Unified Spacememory Network: from Cosmogenesis to Consciousness." Neuroquantology, 2016, 14(4).
[16] H. J. H. Geesink and M. Schmieke, “Organizing and Disorganizing Resonances of Microtubules, Stem Cells, and Proteins Calculated by a Quantum Equation of Coherence,” JMP, vol. 13, no. 12, pp. 1530–1580, 2022, doi: 10.4236/jmp.2022.1312095.